Direction 1 – Cardiac Biomechanics and Regeneration
Epicardial Prestrained Confinement as a Heart Ventricle Protection Interface
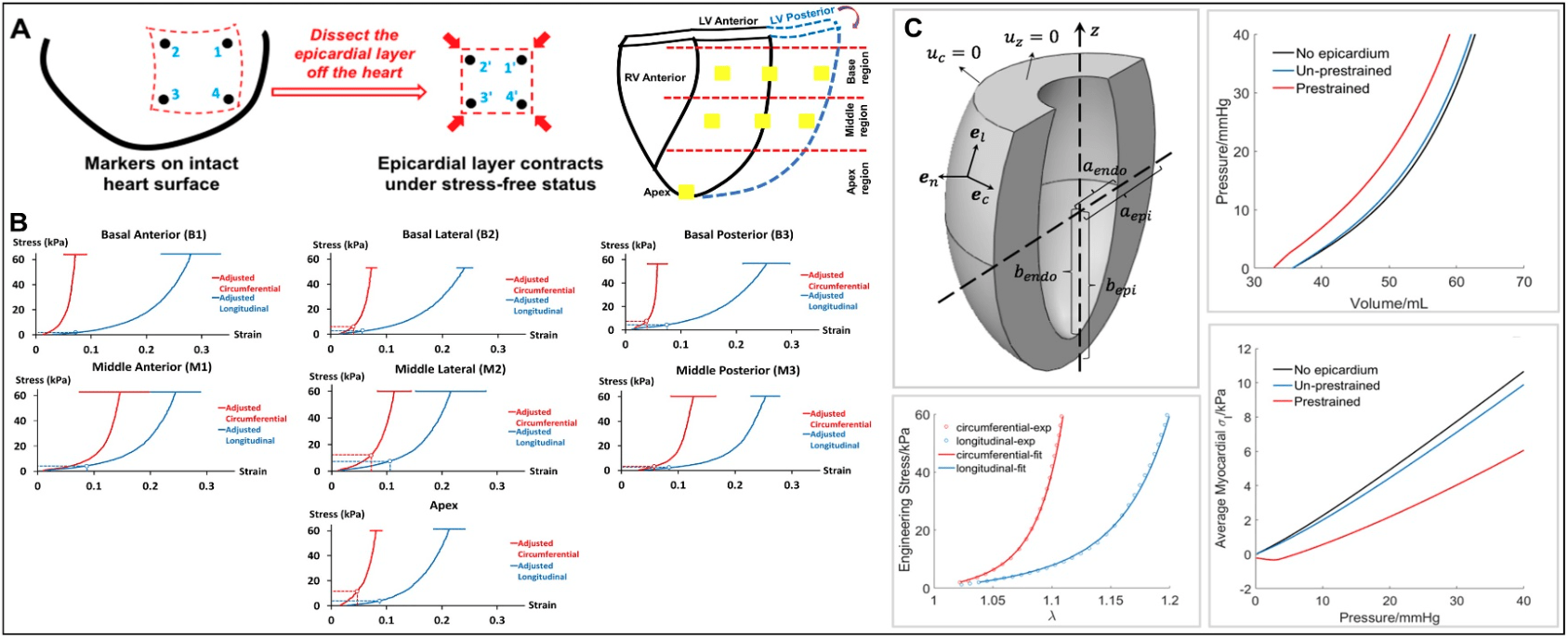
Collaborating with Dr. Huajian Gao at Brown University and Dr. Pietro Bajona at UTSW, we are the first team that reveals an important phenomenon of the epicardial layer of the heart (the surface layer of the heart wall). We discovered and demonstrated that the epicardial layer, rich in elastin, acts like a prestrained ‘balloon’ that wraps around the heart and functions as an extra confinement and protection interface. This finding will help design novel biomimicking materials or prosthetic devices to target the maintenance/recreation of this ventricle confinement interface for heart failure treatment.
Cardiac ECM Biomechanics in Heart Regeneration
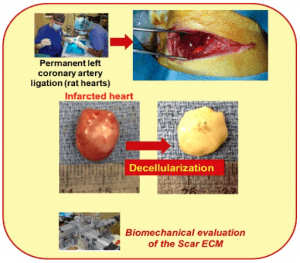
Myocardial infarction (MI) affects more than 8 million Americans, causing massive heart cell death and heart function to decrease. As a promising strategy, stem cell therapy delivers cells to damaged areas (scar tissues) to revitalize the infarcted heart. Unfortunately, the success rate for stem cells differentiating into cardiac muscle cells is extremely low, limiting the great potential of stem cells for cardiac repair and regeneration. In this project, we are investigating the underlying mechanisms that hinder the regenerative potential of stem cells in scar tissue of the damaged hearts. (Collaborators: Dr. Ryan Butler, Dr. Andrew Claude/MSU)
Cardiac Tissue Engineering via Acellular Myocardial Scaffolds
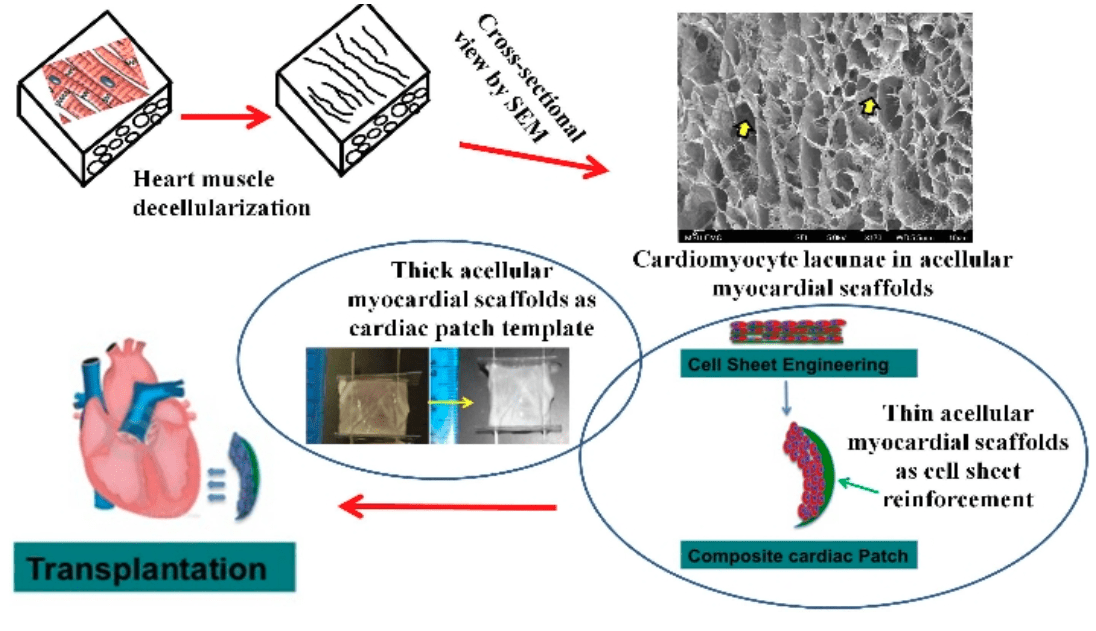
In this project, we assess whether the decellularized myocardial scaffold, along with coordinated multifaceted stimulations, promotes the differentiation of stem cells towards cardiomyocyte phenotype. The knowledge gained from the proposed research will help us better understand fundamental bioengineering in the development of a thick cardiac patch and benefit future exploration of whole-heart reconstruction using the scaffold template from a large animal heart. (Collaborators: Dr. Ge Zhang/University of Akron, Dr. Yi Hong)
Topic 4: 3D Heart Muscle Fiber Disruption Reconstructed via Diffusion Tensor–MRI
Our study is the first attempt to characterize the disruption of the 3D myocardial fiber structure in an area of myocardial infarction and correlate this disruption with the structural and biomechanical changes of the infarcted myocardium. We have successfully created myocardial infarction in a pig model with a balloon catheterization technique, and developed a diffusion tensor magnetic resonance imaging (DT-MRI) protocol to image the infarcted heart explants and a 3D image reconstruction algorithm to reconstruct the muscle fiber architecture. We have demonstrated that the DT-MRI is able to visualize the disruption of the 3D myocardial fiber structure in the infarcted regions of heart explants. (Collaborator: Dr. Song Zhang/MSU)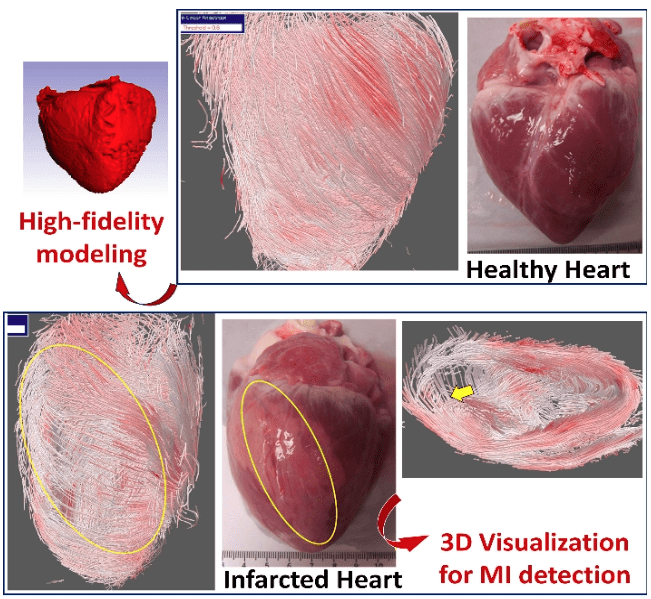
Direction 2 – Heart Valve Biomechanics and Bioengineering
The Fundamentals of Heart Valve Tissue Biomechanics
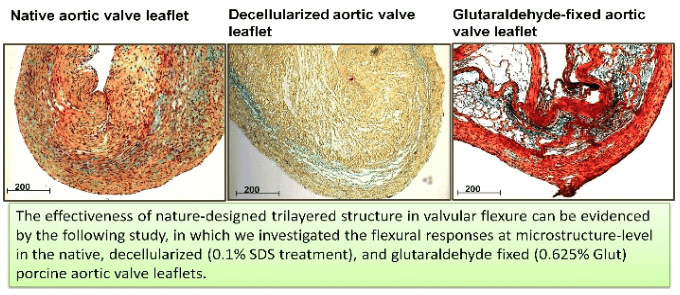
We are currently investigating the ultrastructural mechanism, which minimizes creep and allows valve leaflets behaving as quasi-elastic” biological materials” in a manner that favors valvular function. Our other major contribution in heart valve mechanics is to determine the intrinsic mechanisms of the structural degeneration of ECM due to various decellularization processes, as well as investigate the effects of decellularization on the fatigue resistance of valvular tissues. (Collaborator: Dr. Michael S. Sack/University of Texas at Austin)
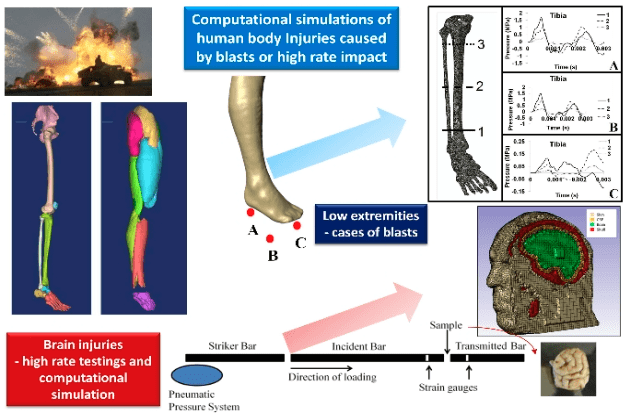
Direction 3 – Injury Biomechanics and Human Body Simulation
In this research direction, we are developing a high fidelity human body mesh with anatomic details of important organs/tissues using 3D reconstruction software and physically-based constitutive models that capture the formation of tissue defects and their dependence on strain level and strain rate. The human body mesh has been integrated into finite element (FE) models that are subsequently subjected to blast and crash impact events. The knowledge-based on the integrated simulations will be applied to facilitate the development of safety countermeasures for combat vehicular systems and solider protective equipment. (Collaborators: Dr. Lakiesha Williams, Dr. Raj Prabhu, Dr. Mark F. Horstemeyer/MSU)